Product Registration
Everva works with various governments, health ministries, agencies and NGO’s.

























Registration
Pharmaceutical product registration is a demanding task in regulated, semi regulated and rest of world countries. Although the requirements are harmonized in regulated countries by CTD (Common technical document) filing, yet others have enormous diversity in requirements. International conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human use brought regulatory authorities and pharmaceutical industries of Europe, Japan and US together for various aspects of drug registration. Similarly, countries from Asia pacific and gulf are in process of harmonization with mutual concern as The Association of Southeast Asian Nations (ASEAN) and Gulf Co-operation Council (GCC). The optimization in requirements is mandatory and can be judged by the incidence of higher cost involved in availability of drugs, research and development facilities. For better treatment safety and efficacy for the drugs must be justified and rationalize for public security. The quality, safety and efficacy data has its own importance in the registration dossier. The commercial significance of markets is increasing globally. It is vital for pharmaceutical industry to cope with the regulatory requirements for betterment of public and to ensure their place in the market.
Our Drug Regulatory team
The Main responsibility of any Government drug regulatory agency is to ensure that the products accessible to their citizens in terms of quality, quantity and efficiency meet the required standards. We have a dedicated registration department that deals with all the matters regarding registration and allows us to register our product in different countries at record time. Our regulatory services include regulatory intelligence, regulatory strategy, regulatory operations, product registration and life cycle management.
Our distributors possess full trust in us to complete the registration. We stick with our distributors, even if take months or years until the products are approved. Product registration varies from country to country as requirements keep varying. Whether in any range of our products, we see the process to the very last step till the end.
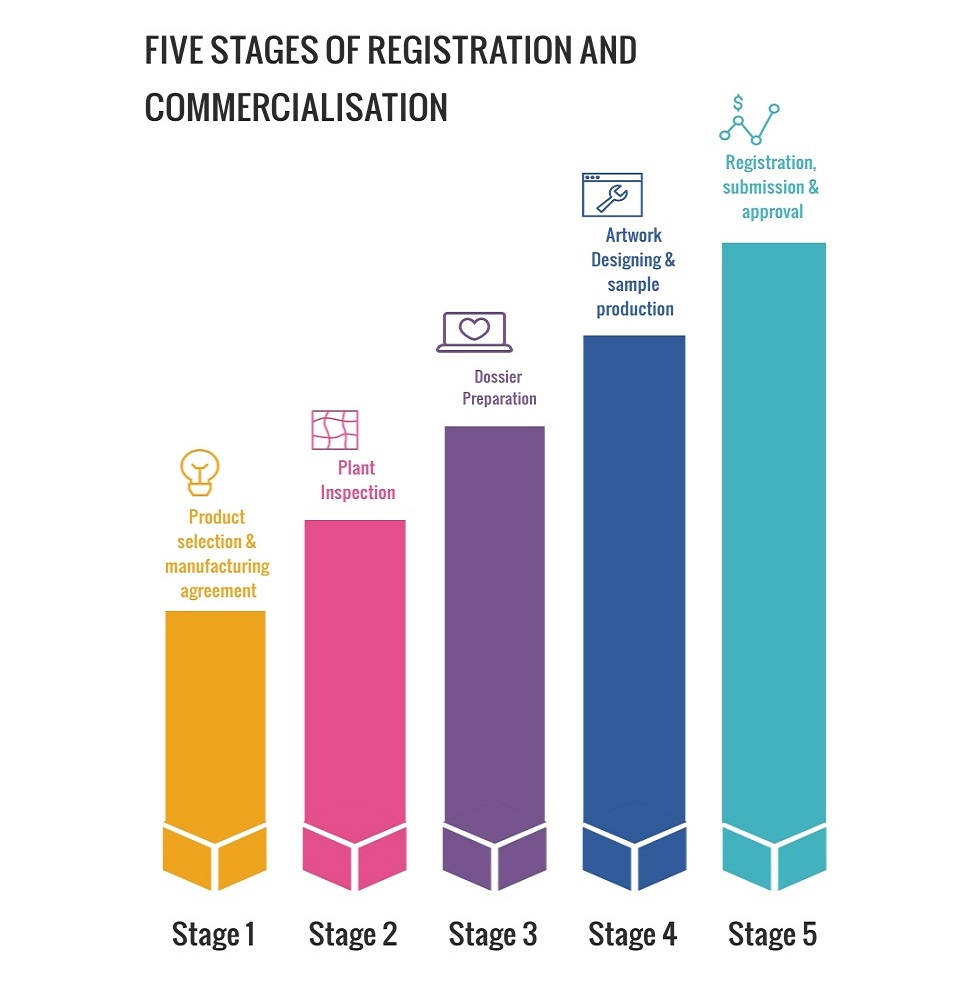
Our Registration Process
Product selection & manufacuring agreement
Products are carefully selected by the distributor and an agreement is drafted based on these products, product specifications and terms of cooperation. The aim of this agreement is complete transparency between both the sides resulting is a long term win-win association.
Plant Inspection
Plant inspections are a part of overall drug quality assurance system. Many countries conduct plant inspection in order to ensure the quality of the products. We keep ourselves ready for such plant inspections by conducting regular internal and third party audits. Everva supports such audits by different drug regulatory authorities and our team ensures that the requirements are met.
Dossier Preparation
Pharmaceutical Dossier is a critical part of product registration process. Different Regulatory Authority published their Standard format according to country Guidelines.
Common Pharmaceutical Dossier which is widely used in the Pharmaceutical Industry are:
- CTD Dossier
- ACTD Dossier
- eCTD Dossier
- Country specific registration dossier
Dossier consists of the collection of technical and administrative data to prove the safety and efficacy of the products. Everva has a strong intellectual wealth generated in the form of CTD dossiers. Our experienced drug regulatory team has an exposure in handling the European, Australian, Latin American, Swiss, ASEAN and other authorities. Our team can skilfully handle all matters related to the registration of our products.
Artwork designing & sample production
Artworks are carefully designed based on the inputs from our distributor and market demand. Appearance of a product can have aesthetic and symbolic value for customers and it also communicate the first impression of the product.
Samples and working standards are required by many drug regulatory agencies for testing and awarding the registration of the products. We can provide the samples of required specification to our distributors for submission and getting the registrations on time.
Registration submission & approval
We ensure timely submission of the dossier, samples and working standards so we get awarded in the form of product registration.
Products are carefully selected by the distributor and an agreement is drafted based on these products, product specifications and terms of cooperation. The aim of this agreement is complete transparency between both the sides resulting is a long term win-win association.
Plant inspections are a part of overall drug quality assurance system. Many countries conduct plant inspection in order to ensure the quality of the products. We keep ourselves ready for such plant inspections by conducting regular internal and third party audits. Everva supports such audits by different drug regulatory authorities and our team ensures that the requirements are met.
Pharmaceutical Dossier is a critical part of product registration process. Different Regulatory Authority published their Standard format according to country Guidelines.
Common Pharmaceutical Dossier which is widely used in the Pharmaceutical Industry are:
- CTD Dossier
- ACTD Dossier
- eCTD Dossier
- Country specific registration dossier
Dossier consists of the collection of technical and administrative data to prove the safety and efficacy of the products. Everva has a strong intellectual wealth generated in the form of CTD dossiers. Our experienced drug regulatory team has an exposure in handling the European, Australian, Latin American, Swiss, ASEAN and other authorities. Our team can skilfully handle all matters related to the registration of our products.
Artworks are carefully designed based on the inputs from our distributor and market demand. Appearance of a product can have aesthetic and symbolic value for customers and it also communicate the first impression of the product.
Samples and working standards are required by many drug regulatory agencies for testing and awarding the registration of the products. We can provide the samples of required specification to our distributors for submission and getting the registrations on time.
We ensure timely submission of the dossier, samples and working standards so we get awarded in the form of product registration.
Our Registration Process
Products are carefully selected by the distributor and an agreement is drafted based on these products, product specifications and terms of cooperation. The aim of this agreement is complete transparency between both the sides resulting is a long term win-win association.
Plant inspections are a part of overall drug quality assurance system. Many countries conduct plant inspection in order to ensure the quality of the products. We keep ourselves ready for such plant inspections by conducting regular internal and third party audits. Everva supports such audits by different drug regulatory authorities and our team ensures that the requirements are met.
Pharmaceutical Dossier is a critical part of product registration process. Different Regulatory Authority published their Standard format according to country Guidelines.
Common Pharmaceutical Dossier which is widely used in the Pharmaceutical Industry are:
CTD Dossier, ACTD Dossier, eCTD Dossier, Country specific registration dossier
Dossier consists of the collection of technical and administrative data to prove the safety and efficacy of the products. Everva has a strong intellectual wealth generated in the form of CTD dossiers. Our experienced drug regulatory team has an exposure in handling the European, Australian, Latin American, Swiss, ASEAN and other authorities. Our team can skilfully handle all matters related to the registration of our products.
Artworks are carefully designed based on the inputs from our distributor and market demand. Appearance of a product can have aesthetic and symbolic value for customers and it also communicate the first impression of the product.
Samples and working standards are required by many drug regulatory agencies for testing and awarding the registration of the products. We can provide the samples of required specification to our distributors for submission and getting the registrations on time.
We ensure timely submission of the dossier, samples and working standards so we get awarded in the form of product registration.


